“It has been recognized that hydrogen bonds restrain protein molecules to their native configurations, and I believe that as the methods of structural chemistry are further applied to physiological problems it will be found that the significance of the hydrogen bond for physiology is greater than that of any other single structural feature.” -Linus Pauling, The Nature of the Chemical Bond
Introduction
Much has been said of the Anthropic Coincidences, the special values of physical constants and laws of nature that enable our universe to support carbon-based life. Even more remarkable are the wonderful physical-chemical processes that sustain the life of all living things, and that these processes are made possible by hydrogen bonding.
In a previous post, The Theology of Water, I discussed the unusual properties of water, properties that are due to hydrogen bonding, properties that enable an environment friendly to life as we know it on earth. In this post I want to explore the nature of the hydrogen bond and its significance for molecular biology and physiology.
Hydrogen bonding plays a role in biochemical reactions, in anti-body mechanisms, in all of molecular biology and, most importantly, in how DNA acts as a blueprint for the synthesis of proteins. A book would be needed to tell about all of these things, so I'll focus on two essentials—the nature of a hydrogen bond and its role in the structure and function of DNA.
What Are Hydrogen Bonds?
Let's imagine God thinking when He is about to design what nature is to be:
“Now I want chemistry to have not only strong interactions between atoms, but also gentle ones: so that complicated structures can unfold and rewind easily, and so that big and small molecules can come together and join for reactions and go apart readily--Velcro or a zipper, not glue or nails. What should I use? I have it--a hydrogen bond.”
(Note: please don't criticize me for heresy here--I'm using a semi-literary device to make a point. I know God holds an infinite number of thoughts and plans simultaneously in His infinite mind.)
Below are shown the types of hydrogen bonds important in molecules of biological interest.
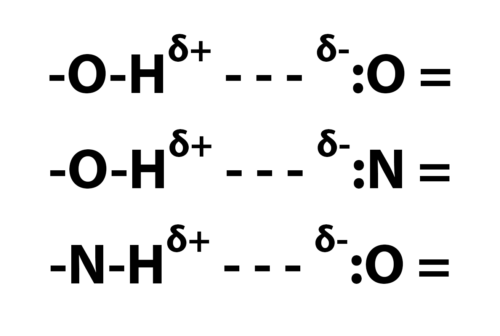 The single dashes represent single bonds; The = signs, double bonds; The - - -, hydrogen bonds; The " : ", lone pair electrons; Superscripts, delta plus and delta minus, small net positive and negative charges.
The single dashes represent single bonds; The = signs, double bonds; The - - -, hydrogen bonds; The " : ", lone pair electrons; Superscripts, delta plus and delta minus, small net positive and negative charges.
Here's the basic idea: H (hydrogen) bonded to O (oxygen) as in H-O-H (water) shows a slight positive electrical charge; :O, oxygen with a pair of unbonded (lone) electrons, shows a slight negative charge. Similarly, :N (nitrogen), with a pair of lone electrons shows a slight negative charge, and N-H, hydrogen bonded to nitrogen, show a slight positive charge. There is an electrical attraction between these small positive and negative charges; also, as nmr experiments have recently shown, there is a contribution from chemical bonding (sharing of electrons) to hydrogen bonding, so that it is more than simple electrostatic interaction.
Hydrogen bonds energies are about 1/20 to 1/30 the value of ordinary covalent bonds, so the hydrogen bonds can be broken much more easily than covalent bonds; for example the O-H bond energy is about 430 kJoules/mole, whereas the O-H - - - :O hydrogen bond energy is only 21 kJoules / mole
Hydrogen Bonding and Base Pairing in DNA
Watson (or was it Crick?) in a moment of insight noticed that organic bases (nitrogen containing molecules bound to sugar pieces in nucleotides such as DNA, RNA) matched each other by hydrogen bonding like pieces in a jig-saw puzzle. They could thus stabilize a helical structure, by links across the spiral, as shown below.

The base pairs are shown below:

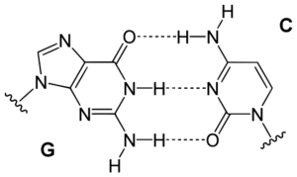
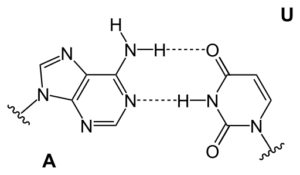
These bases are attached to sugar-type pieces, which in turn have phosphate groups on them that form the links between base units. The hydrogen bonds linking base pairs are strong enough to hold together the two DNA strands in the spiral helix, but weak enough that they can be "unzipped" by the mild chemical action of an enzyme, RNA polymerase, which yields messenger RNA.
Alphabet Blocks for Building Proteins
Before discussing the mechanism by which DNA enables protein synthesis, a few remarks are in order about the bases as letters in a word, words which encode which amino acids are used as building blocks in the protein.
In this process a linear combination of three bases is used to encode which amino acid is put into a protein. So we can regard the bases as letters and the combination of three bases as a three letter word; the three letter word is called a "codon". There are four bases (1) so there are 4^3 = 64 possible codons. There are 20 amino acids found in proteins, plus codons for beginning and ending protein synthesis, so that incorporating a given amino acid may be encoded by several codons, i.e. there is a redundancy. See here and here for tables showing specific codon / amino acid relations.
Gene Expression - Transcription and Translation
I'll give just a brief summary here of gene expression--transcription and translation. Gene expression is the term used to describe the enzyme catalyzed synthesis of messenger RNA, mRNA, from a DNA template, and the process by which mRNA then is used to assemble proteins in the cell from amino acids. Detailed accounts are given in the linked web sites and videos.
STEP 1: transcription—The enzyme, RNA polymerase, unzips the DNA double strand and attaches complementary bases to single strand RNA. See here and here (2). Note that the RNA polymerase is a large protein, much bigger than the DNA strand. Also note that one strand of the DNA serves as a "template"--bases complementary to bases in the template strand are linked, e.g. G to C, C to G, A to T, U to A, and as they're linked they detach to yield mRNA. See this nice flash animation for a more detailed description of this process.
STEP 2: translation--mRNA leaves the cell nucleus, goes into the cytoplasm where it attaches to a ribosome, where protein synthesis occurs. In the process transfer RNA molecules are sent by the ribosome to attach specific amino acids, coded by the m-RNA, to form a protein.
Commentary
The description above is extremely concise--a lot is left out, so I strongly urge the reader to look at the recommended links, animations and explanations (3). Summarizing gene expression in one paragraph is much like trying to do that for the Bible, Old and New Testaments.
What amazes me is that molecular biologists and those who deal with gene expression, and all the other wonders of molecular biology don't paraphrase Psalm 19A: "DNA declares the glory of God, and gene expression shows forth the work of His hands." Certainly the hydrogen bond, which plays a crucial role in these processes, neither too weak nor too strong, is a marvel in itself. God's providence in molecular biology is as marvelous as it is in physics.
Notes
(1) Uracil replaces thymine in the RNA messenger and is encoded by the complement of thymine, adenine--see the section on gene expression. The presumed explanation for this replacement is greater chemical stability of uracil compared to thymine.
(2) Scroll down to get to the animation and click on the arrow to start it.
(3) There are a series of posts from Biologos that explain in clear detail the gene expression process. The link above goes to the first in the series.

