“To answer the question ‘To be or not to be?’ we cannot turn to a science textbook." —Fr. Stanley Jaki, The Limits of a Limitless Science.
The quote from Fr. Jaki says it all—there are questions that science cannot answer by the very nature of how science works, including:
- Why should science explain?
- Why should the laws of nature “be written by God in the hand of mathematics” (Galileo)?
- Where does “The unreasonable effectiveness of mathematics in the natural sciences” (Eugene Wigner) come from?
I’ll discuss possible answers to those questions in this and the following articles (or why there might be no answers).
Philosophical Background
As a one-time practicing physicist, I never bothered about why science works. It was only after retiring that I began to wonder about the philosophical ground on which science rests. Delving into texts, I found there were two main camps of philosophers of science: realist and antirealist. A summary of my blog post about the realists and antirealists, Tipping the Sacred Cow of Science—Confessions of a Science Agnostic, is given below.
The Realists
“Scientific realism … is a quite limited claim that purports to explain why certain ways of proceeding in science have worked out as well as they (contingently) have.”
—Ernan McMullin, A Case for Scientific Realism”
Scientists and philosophers who are “scientific realists” believe there is an underlying reality corresponding to a scientific description. Thus, they believe that the so-called “God particle,” the Higgs boson, is real—even though its existence is inferred from theory and high energy particle scattering data.
It should not be surprising that most scientists are scientific realists. Why would a molecular biologist spend his life exploring the mysteries of the human genome unless he/she believed that this was a “true” explanation of how molecular chemistry expresses itself in heredity and our biology? Why would I (when I was an MRI physicist) have spent too many hours per day wondering how the state of biological tissue affected the environment of water protons unless I believed that protons existed and that their magnetic properties depended on physical interactions with their tissue environment? To most of us scientists, it’s more than a game; it’s a passionate desire to know how nature operates and—for those of us who believe in God—to understand His wisdom in Creation.
The Anti-Realists
“The fundamental laws of physics do not describe true facts about reality. Rendered as descriptions of facts, they are false; amended to be true, they lose…explanatory force.”
—Nancy Cartwright, “How the Laws of Physics Lie”
Anti-realist philosophers deny the fundamental reality of scientific theories/laws/entities and claim that science is validated only empirically, by the truth or falsity of the predictions derived from scientific theories. For these “anti-realists,” there are no scientific laws, and the reality of theoretical entities–quarks, gluons, etc.–is problematic. According to them, scientific theories do not mirror reality, veiled or unveiled. Their philosophical positions are illustrated by Nancy Cartwright’s How the Laws of Physics Lie, and Bas van Fraassen’s The Scientific Image discussed below.
Nancy Cartwright does not believe that fundamental, theoretical scientific equations are true (e.g. Maxwell’s equations, the Schrodinger equation), although she does believe that theoretical entities are real (e.g. the electron, quarks), and that phenomenological equations are true (relations empirically rather than theoretically derived, such as Poiseuille’s Law of viscous flow). Her reason for disbelief, supported by a number of examples, is stated in the quote above. The equations have to be amended, supplemented, supplied with empirical fudge factors, or require conflicting mathematical prescriptions to describe real situations. I won’t go over all her examples (Snell’s Law of refraction, Crooke’s radiometer, quantum damping, and the BCS theory of superconductivity) but only say they are well chosen to demonstrate her knowledge of mathematical physics.
Bas van Fraassen believes that a scientific theory can be judged to be true if it is empirically verified, and only by that test. Here I agree with him—to an extent. He believes that theory does not correspond to a real-world, discoverable by science that would be a metaphysical assumption, a truly bad thing for an empiricist philosopher. With this judgment, I might disagree. Van Fraassen also argues that theory cannot be evaluated as the best explanation for phenomena. (The ether is one example of an incorrect but plausible explanation for how electromagnetic waves vibrate.) The function of theory is “to save the phenomena,” i.e. to give a concise description of real phenomena that is empirically verified. He also limits that which might be described as truly real, an “observable,” to that which can be directly observed (in principle), such as Jupiter’s satellites. That which can only be detected by the use of instruments—chromosomes, electrons, quarks—would not be an “observable” and thus not “real.”
The anti-realists, looking at the history of science, sees failed theories and theoretical entities disproved by experiments—such as phlogiston by Count Rumford’s cannon-boring experiments and the ether by the Michelson-Morley experiment. On the other hand, one could argue that theory is converging to the truth–the Higgs boson confirmed by the CMS experiments—even though that convergence is an article of faith.
I myself have faith in science as a partial mirror of reality. I believe that the world that God created must be an orderly and intelligible world—not the “dappled world” of Nancy Cartwright, with one set of scientific laws here and another there (my music is Bach, not Cage). God gave us the intelligence to help us understand his creation; the reality that remains veiled is so because of limits to our intelligence, not because God is irrational.
The Lakatos “Scientific Research Programme”
“Man is not born to solve the problem of the universe, but to find out where the problem begins and then restrain himself within the limits of the comprehensible.” —J.W. von Goethe, as quoted in “The Homiletic Review”
If you do a man-in-the-street survey, asking “How does science work?” you’ll get many different answers. Here are some from an adult education class I taught on “Science and the Church” (actually, I supplied the last two answers):
- Finds theories to explain everything;
- Formulates a hypothesis, tests the hypothesis by experiment;
- Finds an unusual experimental result, formulates a theory to explain the result;
- Finds a theory that will explain a body of experimental knowledge;
- Finds theories that could be proved false by suitable experiments;
- Finds which theories are the most elegant and are also consistent with experimental results;
- Depends on a scientist’s presuppositions and assumptions;
- Is “reductionist”, i.e. attempts to reduce phenomena and the objects comprising these phenomena to the smallest components and the scientific laws governing the action of these components (for example, intelligence can be reduced to biochemical and electrical events on the molecular level);
- Establishes a research program consisting of a network of hypotheses and experimental data: core theory, based on inner core principles, linked to secondary theories and results (Lakatos Scientific Research Program—see below).
The correct answer is “all the above,” depending on the scientist and his/her research focus. However, I believe the last answer, the Lakatos Scientific Research Program, gives the best, the most complete description of how science is carried out. This “Scientific Research Programme” can be thought of as a sphere:
- Inner Core (fundamental principles): not theories, but principles to which theories have to adhere; these principles are assumed because they seem obvious and confirmed generally by our experience (for example, The First and Second Laws of Thermodynamics). But, as we’ll see below, there are occasions when these fundamental principles are modified or violated.
- Primary Shell (fundamental theories): surround the core of fundamental principles (e.g. thermodynamics, general relativity, quantum mechanics).
- Other Shells (auxiliary theories): surround the shell of fundamental theories; such auxiliary theories are derived from the primary theories and other auxiliary theories (e.g., MRI, chemical bonding, heat transfer).
- Outermost Shell (experimental facts or data): the interplay between the shells and core is described in the diagram below and illustrated below by several examples in Case Studies of How Science Works.
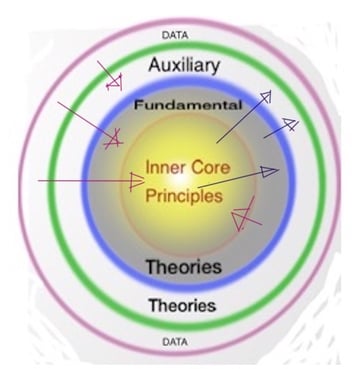 Diagram “Lakatos Experimental Research Program”
Diagram “Lakatos Experimental Research Program”
Black arrows: From inner core to theories
Red arrows: Feedback from theories, facts
I’ll first show how science does work with some historical examples; I’ll then discuss how the Lakatos scheme can be applied to these examples.
History: How Science Works
Thermodynamics: Cannon Boring to Heat Not Conserved
 Count Rumford’s Cannon-Boring Experiment–Making Water Boil
Count Rumford’s Cannon-Boring Experiment–Making Water Boil
In 1798 Benjamin Thompson, Count Rumford, submitted a paper to the Royal Society about his experiments in which boring a cannon could make water boil, and boring with a blunt instrument produced more heat than with a sharp one (more friction with the blunt). The experiments showed that repeated boring on the same cannon continued to produce heat—so. clearly, the heat was not conserved and, therefore, could not be a material substance.
This experiment disproved the then prevalent theory of heat: that it was a fluid transmitted from one thing to another, “the caloric.” The results validated another theory of heat, the kinetic theory, in which heat was due to the motion of atoms and molecules. However, the kinetic theory, despite Rumford’s groundbreaking experiment, still did not hold sway until years later, after James Joule showed in 1845 that work could be quantitatively converted into heat.
Thermodynamics: James Joule: Work to Heat
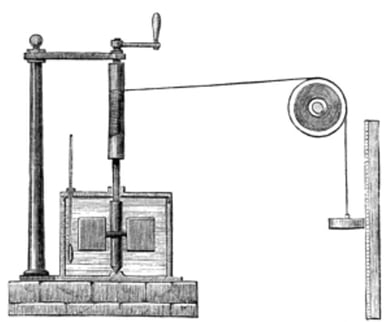 Joule’s Apparatus: Measuring Equivalent Of Heat To Work
Joule’s Apparatus: Measuring Equivalent Of Heat To Work
from Wikimedia Commons
As the weight falls, the potential energy of the weight is converted into work done (a paddle stirs the water in the container against a frictional force due to water viscosity). The temperature rise, corresponding to a given fall of weights (work done), yields the amount of heat rise (in calories) of the known mass of water. Since the temperature rise is very small, the measurements have to be very accurate.
It took 30 to 50 years after Joule’s definitive experiment (and subsequent refinements and repetitions) for the kinetic theory of heat—heat caused by the random, irregular motion of atoms and molecules–to be fully accepted by the scientific community. James Clerk Maxwell published a paper in 1871 called “Theory of Heat“. This comprehensive treatise and advances in thermodynamics convinced scientists to finally accept that heat was a form of energy related to the kinetic energy (the energy of motion) of the atoms and molecules in a substance.
Contemporary: Experimental Tests of Fundamental Theories
Below, I give links to examples of modern experimental tests of ground-breaking primary and secondary theories in various fields of science (some are discussed below in more detail and in other essays:
- Special Relativity
- General Relativity
- Plate Tectonics
- The Genetic Code
- The Higgs Field to Endow Particle with Mass (see also: “God, Symmetry and Beauty in Science: The Standard Model and the Higgs Boson“)
Applying The Lakatos Scheme
Rumford and Joule’s Experiments on Heat and Work
The core principle involved in the caloric theory of heat was the conservation of caloric (since it was a substance). Count Rumford’s cannon-boring experiments showed that the more the cannon was bored, the more heat was produced; therefore the supply of heat in the cannon was inexhaustible and clearly not conserved. A core principle involved in Joule’s experiment is the First Law of Thermodynamics: conservation of energy, with heat and work as forms of energy. Note that this conservation principle is linked to the fundamental theory of thermodynamics developed in the middle of the 19th century and earlier—theories of classical mechanics developed in the 18th century and early 19th century.
Einstein’s Special and General Theories of Relativity
Einstein’s two theories of relativity are striking examples of how theory influences fundamental principles, or perhaps more accurately, how fundamental principles are proposed as a basis for general theories. His theory of special relativity introduced the following new general principles:
- the laws of physics are the same for systems (“frames of reference”) moving at constant velocity (i.e., “inertial systems”);
- the speed of light (in vacuum) is constant, regardless of the speed of source or receiver;
- neither energy nor mass is conserved but only mass + energy (from E= mc²)
His general relativity theory introduced the “equivalence principle,“ that inertial and gravitational mass are the same. In everyday terms, this principle says that a person (mass m) in an elevator accelerating upward experiences a force holding him to the floor due to earth’s gravitation, mg, plus a force due to the acceleration of the elevator, ma. This is the same force that the person would experience on a planet where the gravitational acceleration would correspond to g+a, or in a spaceship accelerating at a rate g+a.
Recently Einstein’s theory of general relativity has been confirmed again from LIGO measurements of gravity waves.
Is Parity Conserved? Right- and Left- Handedness
Parity refers to mirror symmetry. For example, many organic molecules are either right- or left-handed (see the illustration below of two amino acids, constituents of proteins: COOH is the organic acid group, NH2 is an amino group, C is the central carbon, and R represents a general group attached to the carbon).
Biological molecules can be chiral either as a whole or with respect to the constituent parts. For example, amino acids found in nature are left-handed; sugars found in nature are right-handed; DNA as a whole has a right-handed spiral (helix). The question of why only one kind of handedness for biological molecules came about has fascinated chemists and biologists since the time of Pasteur 150 years ago. There are recent theories to explain this, but they are to some extent conjectural.
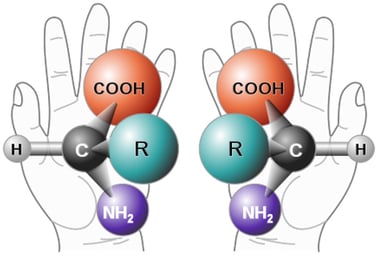 Left- and right-handed molecules (chiral molecules).
Left- and right-handed molecules (chiral molecules).
These amino acids are mirror images of each other.
from Wikimedia Commons
Conservation of parity (handedness) had been a fundamental principle of physics until the late 1950’s when a proposal to test it for nuclear weak force interactions–e.g. beta decay of Co-60 nuclei–showed that it was violated. Since that time a conservation principle, CPT symmetry, linking parity (P) with charge (C), and time-reversal (T) has been found to hold.
Final Thoughts
I’ve shown (I hope) that science is neither pure theory nor purely a collection of facts. The facts have to be interpreted in terms of theory, and not just a particular theory, but a theory that agrees with fundamental scientific principles and general theories. And when new facts or new theories come up, then these principles have to be revised or limited. So, science is not an eternal truth, but a methodology: the truths it determines are subject to change. In this respect, science differs from our Catholic faith; for our faith is not subject to change, but founded on eternal truth.
In the fourth article in this series, I’ll discuss the limits of science and mathematics.


